
CaV1 and CaV2 calcium channels mediate the release of distinct pools of synaptic vesicles
Activation of voltage-gated calcium channels at presynaptic terminals leads to local increases in calcium and the fusion of synaptic vesicles containing neurotransmitter. We demonstrate that synaptic vesicle pools are separated into two pools at C. elegans presynapses. At the center of the active zone, vesicles are tethered to the CaV2 neuronal-type calcium channel. Laterally, vesicles are tethered to the CaV1 muscle-type calcium channels and internal calcium stores by the ryanodine receptor. Vesicles are recruited to these fusion sites by different isoforms of the UNC-13 protein.

Interspecies complementation identifies a pathway to assemble SNAREs
To understand the functional interactions within the core SNARE machinery we adopted an ‘interspecies complementation’ approach in C. elegans. Substitutions of individual SNAREs and Unc18 proteins with those from yeast fails to rescue fusion. However, synaptic transmission could be restored in worm-yeast chimeras when two key interfaces were present: a syntaxin Habc-Unc18 contact site and an UNC-18-SNARE motif contact site. Finally, we demonstrate that the SNARE and UNC-18 machinery in the nematode C. elegans can be replaced by yeast proteins and still carry out synaptic transmission, pointing to the deep evolutionary conservation of these two interfaces.
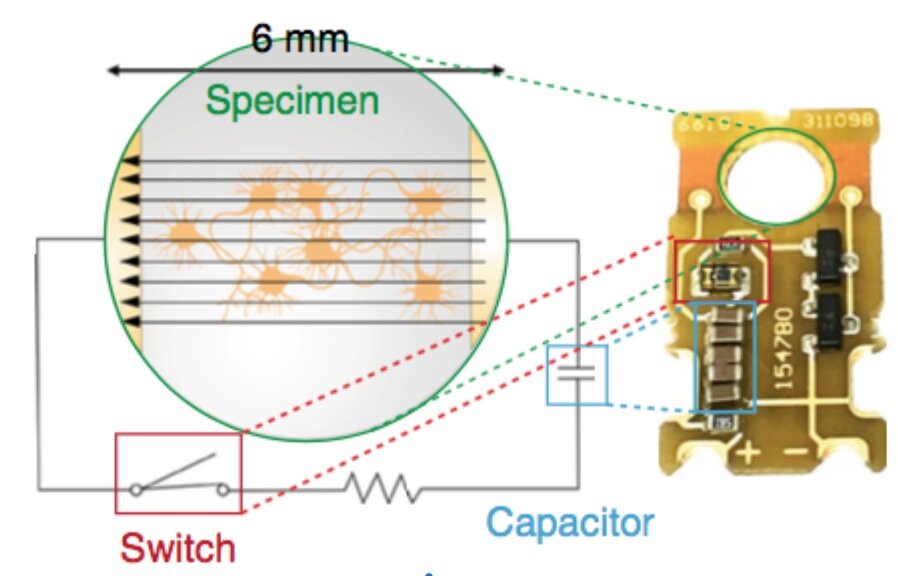
Synaptic vesicles transiently dock to refill release sites.
To characterize the ultrastructure of vesicle docking and fusion at active zones, we developed a method called ‘zap-and-freeze’ to trigger single action potentials by electrical stimulation followed by high-pressure freezing at defined time points. Using this approach, we first characterized the spatial and temporal organization of fusion sites following a single action potential. We observed that during synchronous release, multiple vesicles can fuse per action potential within the same active zone, even in physiological extra- cellular [Ca2+]. Fusions during synchronous release occur through- out the active zone, but are concentrated at the center of the active zone during asynchronous release.
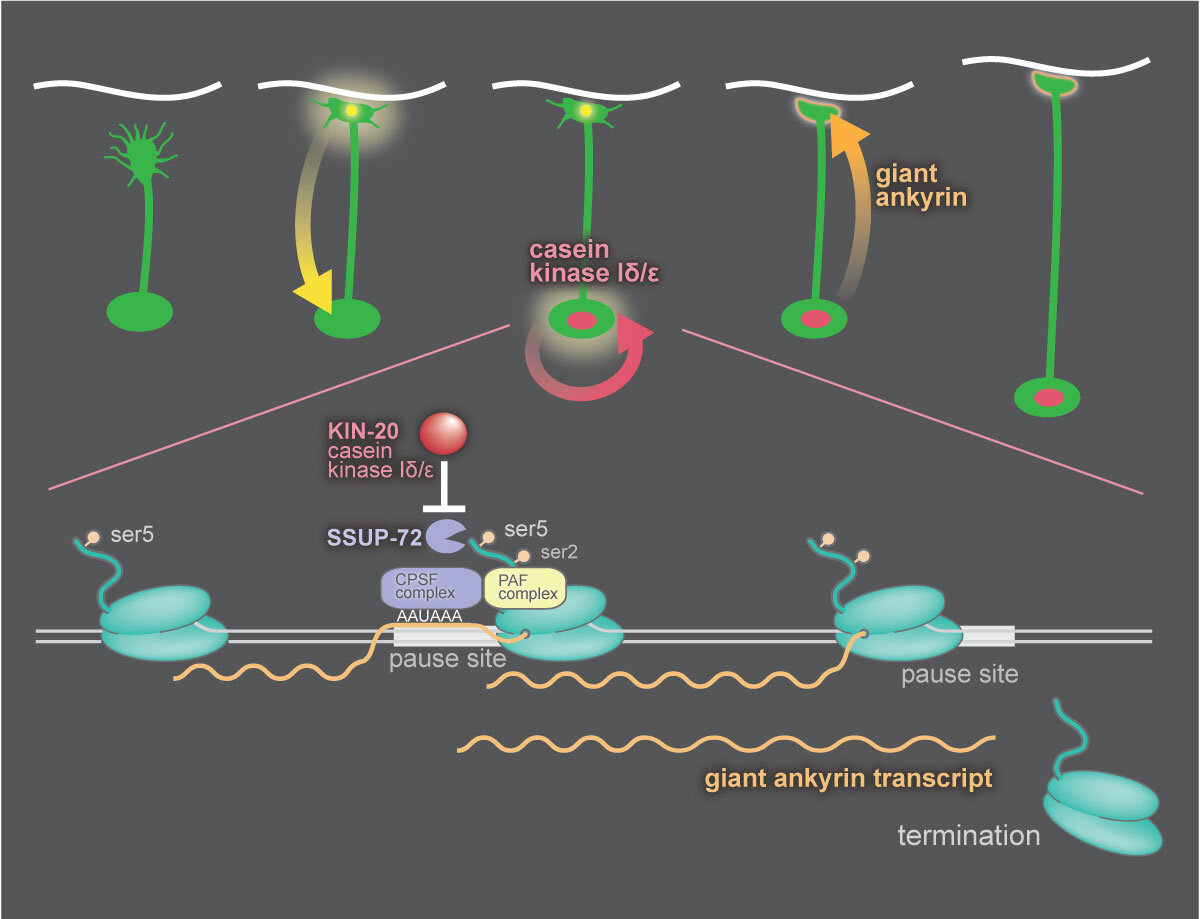
Casein Kinase 1d Stabilizes Mature Axons by Inhibiting Transcription Termination of Ankyrin
LaBella et al.
Developmental Cell 2020
During early development growth cones sprout from neurons and lay down an axon to the neuron’s synaptic targets. After axon outgrowth and synapse formation, the nervous system transitions to a stable architecture that lasts the lifetime of the animal. During this transition, synapses must send a signal to the cell body to reprogram the nucleus. In C. elegans, this transition is mediated by casein kinase 1δ (CK1δ), which modifies the behavior of RNA polymerase II. RNA polymerase has a long C-terminal domain composed of 20-50 repeats of a 7 amino acid sequence. Phosphorylation of serine-5 in this sequence marks actively transcribing polymerase, and removal of this phosphate by the termination factor SSUP-72 initiates transcription termination. CK1δ phosphorylates and inhibits SSUP-72, thereby inhibiting termination. The target of this switch is the gene encoding the cytoskeleton protein Ankyrin. CK1δ activity switches expression from the short to giant isoform of this protein.

Synaptojanin and Endophilin Mediate Neck Formation during Ultrafast Endocytosis
Watanabe et al.
Neuron 2018
Ultrafast endocytosis generates vesicles from the plasma membrane as quickly as 50 ms in hippocam- pal neurons following synaptic vesicle fusion. The molecular mechanism underlying the rapid matura- tion of these endocytic pits is not known. Here we demonstrate that synaptojanin-1, and its partner endophilin-A, function in ultrafast endocytosis. In the absence of synaptojanin or endophilin, the mem- brane is rapidly invaginated, but pits do not become constricted at the base. The 5-phosphatase activity of synaptojanin is involved in formation of the neck, but 4-phosphatase is not required. Nevertheless, these pits are eventually cleaved into vesicles; within a 30-s interval, synaptic endosomes form and are resolved by clathrin-mediated budding. Then synap- tojanin and endophilin function at a second step to aid with the removal of clathrin coats from the regen- erated vesicles. These data together suggest that synaptojanin and endophilin can mediate membrane remodeling on a millisecond timescale during ultra- fast endocytosis.






